
In the dynamic landscape of healthcare and pharmaceuticals, ensuring the safety and efficacy of medications is paramount. This is where Pharmacovigilance comes into play, offering an exciting and fulfilling career path for individuals interested in healthcare and drug safety. In this blog, we’ll dive deep into Pharmacovigilance, its various aspects, career opportunities, and the future it holds.
What is pharmacovigilance and its purpose?
A career in Pharmacovigilance is both lucrative and satisfying. It is a branch of medicine that focuses on identifying, assessing, understanding, and preventing side effects or any other drug-related issues. The safety of medications and medical equipment is monitored by Pharmacovigilance Specialists. They also offer guidance to medical experts on the efficient and safe use of medications. It is a field that is garnering a lot of attention globally and is expanding very quickly.
Pharmacovigilance specialists are in demand more and more as the pharmaceutical industry expands rapidly.
Prerequisites for a Pharmacovigilance specialist include a strong analytical ability, familiarity with drug safety laws, and knowledge of the pharmaceutical sector. It is a great career option for those who are dedicated to making a difference in patient safety and are interested in the medical field. A career in Pharmacovigilance is a demanding but rewarding area that offers excellent work prospects.
Careers in Pharmacovigilance with Different Types of Roles & Responsibilities
Pharmacovigilance experts have a wide range of responsibilities that require them to stay well-informed about the latest developments in medicine, drug safety, and regulatory matters. Different Types Of Roles And Responsibilities Involved In a Pharmacovigilance career are:
1. The Role of a Clinical Research Associate (CRA)
Clinical Research Associates (CRAs) play a pivotal role in pharmacovigilance, especially in clinical trials. CRAs are responsible for analyzing and collecting data from patients, monitoring the safety of drugs and treatments, and reviewing patient records and medical histories. CRAs work closely with regulatory authorities to make sure that any adverse events or reactions are reported promptly and that all safety standards are met.
2. The Role of a Medical Writer
Medical Writers are responsible for creating and maintaining precise and up-to-date scientific documents that provide information on the safety of medicines and other medical products. This includes reports on clinical trial results, adverse events, patient education materials, product labelling, and more. Medical writers must have an in-depth understanding of regulatory requirements as well as medical terminologies to ensure that all documents are precise and compliant with applicable laws. With their expertise, medical writers help to make sure that patients receive effective and safe medications.
3. The Role of a Drug Safety Officer
A Drug Safety Officer (DSO) plays an important role in the world of pharmacovigilance. Their primary responsibility is to make sure that pharmaceutical companies are compliant with the standards and regulations set by regulatory bodies when it comes to drug safety.
They are responsible for monitoring the safety of medical devices and drugs, making sure that they meet regulatory standards and are safe for use. Drug Safety Officers must have an in-depth understanding of interpreting complex data and drug safety regulations to make informed decisions about drug safety. With their expertise, they can help make sure that patients receive safe medications and devices.
4. The Role of a Data Analyst/Scientist
Data Analysts have a crucial role in pharmacovigilance. They are responsible for collecting, analyzing, and interpreting data related to the safety of medical devices and drugs. They use clinical data management to understand the complexities of drug safety and develop strategies to identify potential risks associated with the use of drugs.
The Impact of Pharmacovigilance in the Pharmaceutical Industry
The pharmaceutical business relies heavily on pharmacovigilance to help guarantee that medicines are both safe and effective to use. Before a drug is put on the market, it helps to discover any potential risks connected to it by monitoring and observing drug safety. This procedure aids in preventing any potential harm that a medicine may cause to both patients and medical personnel. We can make sure that medicines are safe to use and that patients receive the greatest treatment by knowing pharmacovigilance and how it affects the pharmaceutical sector.
What are the 4 Stages of Pharmacovigilance?
- Data Collection: The first stage involves collecting data on adverse drug reactions (ADRs) and other relevant information from various sources, including healthcare professionals, patients, and regulatory agencies.
- Data Detection: In this stage, the collected data is analyzed to identify potential safety concerns and trends associated with specific medications.
- Data Assessment: Once potential safety signals are detected, they undergo a thorough assessment to determine their significance and potential impact on public health.
- Risk Management: The final stage involves developing strategies to manage and mitigate the identified risks, which may include updating product labels, conducting additional studies, or even withdrawing a drug from the market if necessary.
Pharmacovigilance Companies in India
India has emerged as a hub for pharmacovigilance services, with numerous companies specializing in this field. These companies provide a wide range of opportunities for professionals looking to build a career in pharmacovigilance.
Top Recruiters in India and Abroad
Some of the top Pharmaceutical companies that employ Pharmacovigilance are as follows:

Read the entire blog on Pharmacovigilance Companies In India.
Choosing the right company is pivotal in shaping a successful career in pharmacovigilance. It’s essential to align your interests and career goals with the company’s mission and values to maximize your potential for growth and impact.
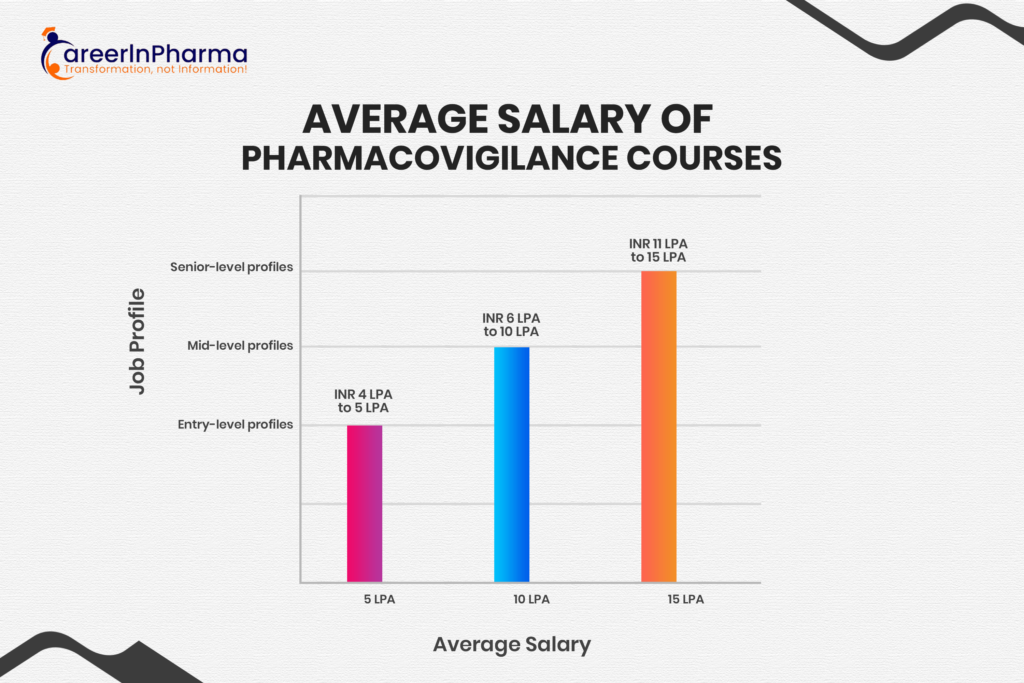
Salary Expectations in Pharmacovigilance
In pharmacovigilance, salary varies depending on many factors such as qualifications, experience, and the employing organization. Entry-level positions can range from INR 4 LPA to 5 LPA, mid-level profiles offer INR 6 LPA to 10 LPA, while senior-level roles may offer salaries of 11 LPA to 15 LPA or more annually. However, it’s important to note that these figures are just averages and can vary significantly based on location and industry demand.
Why Choose Pharmacovigilance?
Pharmacovigilance offers a unique career path for BDS graduates, dentists, MBBS, and Pharma graduates as well as Life Science graduates allowing them to apply their medical knowledge in drug safety and regulatory affairs.
- India ranks as the third-largest global player, producing more than 500 diverse active pharmaceutical ingredients.
- The extensive pharmaceutical and biotech industry with a wealth of skilled professionals
- Government initiatives support and enhance India’s innovative potential.
- Vast opportunities for data mining on drug safety due to the large population.
- Represents 8% of worldwide pharmaceutical production, ranking fourth globally.
- Maintains work-life balance with career growth.
FAQs about Career in Pharmacovigilance
1. What is the Difference Between Drug Safety and Pharmacovigilance?
While drug safety and pharmacovigilance are closely related, they have distinct differences. Drug safety encompasses all activities related to the safety of drugs, including clinical trials and pre-market assessments. Pharmacovigilance, on the other hand, focuses specifically on post-market surveillance, monitoring drugs once they are available to the public to identify and manage adverse events.
2. What Skills are Required for Pharmacovigilance?
- Pharmacovigilance specialists need to have a deep knowledge of medical terminology
- Pharmacovigilance specialists need to have good communication skills to communicate effectively with a range of people, both verbally and in writing.
- Pharmacovigilance specialists must be precise in identifying risks and side effects and develop plans to minimize them.
- Good in Teamwork and Command
3. Is a Career in Pharmacovigilance stressful
The field is under pressure from some factors, including the pharmaceutical industry’s rapid growth, the consequences of cost-cutting strategies and the complexity of the PV system. But it can be financially rewarding and personally satisfying, and it offers a wide range of opportunities for networking, collaboration, and growth. Pharmacovigilance can be a good career choice for people who are passionate about drug safety and healthcare.
Conclusion
Pharmacovigilance is a field that plays a vital role in ensuring the safety and efficacy of medications, making it a rewarding career choice for individuals passionate about healthcare and drug safety. With the ever-expanding pharmaceutical industry and advancements in technology, the future of pharmacovigilance holds immense promise, offering diverse opportunities for professionals to make a meaningful impact on public health. Whether you’re a healthcare enthusiast or an IT expert, a career in pharmacovigilance may be your gateway to a fulfilling and impactful journey.

