
Definition of Pharmacovigilance
Pharmacovigilance is a Greek word derived from pharmaco (drug) and vigilance (watch). It means monitoring a drug during its trial and marketing phases.
According to the WHO, pharmacovigilance is a science and set of activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. Pharmacovigilance in India was initiated way back in 1986 with a formal adverse drug reaction (ADR) monitoring system, under the supervision of the drug controller of India. India joined the World Health Organization (WHO) Program for International Drug Monitoring in 1998 but was not successful.
When was Pharmacovigilancde born?
Pharmacovigilance took birth officially in1961 in Britain, because following the administration of Thalidomide to pregnant women, numerous cases of fetal malformations occurred. This disastrous event marked the start of drug surveillance activity, but there were numerous cases of previously suspected reactions that led to this activity as we know it today as Pharmacovigilance.
Key Events in the History of Pharmacovigilance:
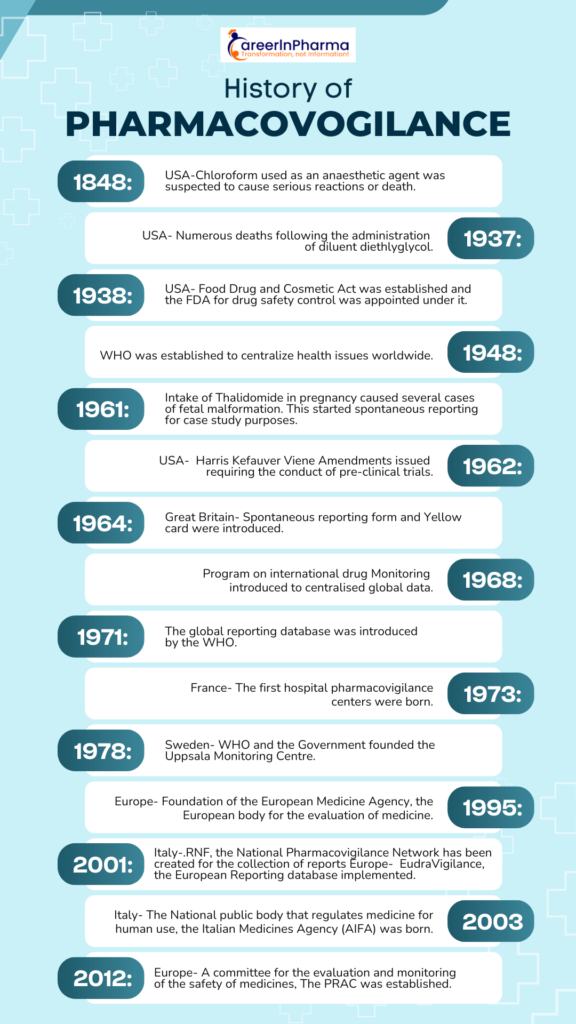
The Chloroform Disaster- 1848
- On 29 Jan 1848, a young girl from the north of England died after receiving chloroform anaesthetic before the removal of an infected toenail. The cause of death was investigated, and it was not possible to identify what killed her and she probably died of lethal arrhythmia or pulmonary aspiration.
The Sulfanilamide Disaster- 1937
- In 1937, 107 deaths occurred in the USA because of the use of sulfanilamide elixir, containing diethyl glycol as the solvent and this incident is called as Sulfanilamide Disaster in the history of PV. Later, the solvent (Diethyl glycol) was identified as the cause of death, but the manufacturing company was not aware of the toxicity at the time of manufacturing.
- Because of the Sulfanilamide Disaster, the Federal Food, Drug, and Cosmetic Act was established in 1938, and the public health system was renovated. As per this act, the safety of the drugs should be demonstrated before their market approval and it introduced the possibility of conducting factory inspections.
Acetylsalicylic acid (ASA) Incidents- 1938

- In 1938, Douthwaite supposed that acetylsalicylic acid (ASA) could cause melena. The study of the gastrointestinal toxicity of ASA showed different outcomes. However, in 1955, it was proved that ASA can cause gastrointestinal diseases and therefore it is currently contraindicated in patients with gastrointestinal ulcers
The Thalidomide Disaster- 1961
- In 1961, a big change in European Pharmacovigilance happened following the tragedy of Thalidomide, and this is known as the Thalidomide disaster in the history of PV.
Thalidomide disaster: It was observed that the incidence of congenital malformations of babies (1.5%) had increased up to 20% in women who had taken thalidomide during pregnancy. At the same time, during a Pediatric Convention in Germany, Dr. Lenz suggested a correlation between malformations and thalidomide, and his suspect was published in a German Journal. The hypothesis of the correlation between the malformations and the intake of the drug was therefore consolidated, and it was withdrawn from the market
Rise of Pharmacovigilance
- In 1964, the “Yellow card” (YC) was structured in the UK. YC is a specific form to compile a spontaneous report of drug toxicity.
- In Europe (1965), the disaster of thalidomide stimulated the development of European legislation with the EC Directive 65/65.
- In 1968, the WHO Program for International Drug Monitoring was instituted, and ten members participated in this program (Australia, the UK, the USA, Germany, Canada, Ireland, Sweden, Denmark, New Zealand, and the Netherlands).
- Many studies of observed adverse drug reactions were conducted between 1968 and 1982.
- In 1992, the European Society of Pharmacovigilance (ESoP) was funded and turned into the International Society of Pharmacovigilance (IsoP). This society aimed to promote Pharmacovigilance and enhance all aspects of the safe and proper use of medicines.
- In 1995, the European Medicines Agency (EMA) was set up.
- In 2001, EudraVigilance was funded. It is the official European database for managing and analyzing information on suspected adverse reactions to medicines that have been authorized for the market or are being studied in European clinical trials
Few Examples of Regulatory Authorities Worldwide:
- The European Medicines Agency (EMA) for countries in the European Union (EU).
- The Food and Drug Administration (FDA) for the United States.
- Marketed Health Products Directorate (MHPD).
- CDSCO (Central Drug Standard Control Organization)
Goals and Importance of Pharmacovigilance
- Early detection of unknown safety problems during Clinical trials.
- Addition of new side effects when the drug is on the market.
- Based on the addition of new events the label (leaflet) gets updated.
- To assess the drug risk/benefit ratio to act on improving the safety
- Communicating information to HCPs and patients.
- Preventing patients from being affected unnecessarily.
- Rational and safety uses of the medicines
Pharmacovigilance In India: Present Scenario & Future Challenges
- Pharmacovigilance in India was initiated way back in 1986 with a formal adverse drug reaction (ADR) monitoring system, under the supervision of the drug controller of India. India joined the World Health Organization (WHO) Program for International Drug Monitoring in 1998 but was not successful.
- Later, the National Program of Pharmacovigilance was launched in 2005 and was renamed the Pharmacovigilance Program of India (PvPI) in 2010. In consideration of having a robust pharmacovigilance system in India, steps were taken.
- The Central Drugs Standard Control Organization established the program in July 2010 with All India Institute of Medical Sciences, New Delhi as the National Coordination Centre, which later shifted to the Indian Pharmacopoeia Commission in Ghaziabad on 15 April 2011. The PvPI works to safeguard the health of the Indian population by ensuring that the benefit of medicines outweighs the risks associated with their use.
- The culture of reporting ADRs has achieved remarkable success, with 250 PvPI-established adverse drug monitoring centers all over India and the provision of training to healthcare professionals.
- The Pharmacovigilance Program of India (PvPI ) is an Indian government organization that identifies and responds to drug safety problems.
- The IPC-PvPI has now become a WHO Collaborating Centre for Pharmacovigilance in Public Health Programs and Regulatory Services.
- The PvPI is trying to address other challenges like counterfeit drugs, antimicrobial resistance, and surveillance during mass vaccinations and other national programs
FAQs about the History of Pharmacovigilance
1. What are the 3 types of pharmacovigilance?
The three main types of post-marketing Pharmacovigilance are:
- Drug safety surveillance.
- Drug abuse and adverse effects monitoring.
- Safety monitoring of new products.
2. When was pharmacovigilance established in India?
Pharmacovigilance in India was established in 2010.
The National Programme of Pharmacovigilance was launched in 2005 and was renamed the Pharmacovigilance Programme of India (PvPI) in 2010.
3. What is another name for pharmacovigilance?
Pharmacovigilance (PV), also known as Drug safety, is the pharmaceutical science relating to the “collection, detection, assessment, monitoring, and prevention” of adverse effects with pharmaceutical products.
4. What is day zero in pharmacovigilance?
“Day Zero” is the date on which a potential adverse drug reaction is identified from medical literature or other sources, starting the clock for regulatory submission of individual case safety reports (ICSRs).

