
A day in the life of a regulatory affairs specialist in pharma is directly related to the culture of innovation, which plays an essential role in the growth and development of the pharmaceutical industry by ensuring the safety of a wide range of items that we all use on a daily basis, from food and beverages to drugs, medical devices and more.
You may think that the role of a pharmaceutical regulatory affairs professional’s responsibility is just to complete paperwork that follows guidelines of the Ministry of Public Health (FDA), and (CDSCO). However, there is so much more regulatory affairs to do in this industry. Regulatory affairs specialists are supposed to provide strategic guidance on the legal and scientific standards that products must follow, thus they must stay up with the ever-changing legislation and guidelines in which their company operates. They must also create and implement new processes or methods that may help fasten the approval of a product (particularly drugs) to result in the growth and profitability of the pharma company. Regulatory Affairs Specialists are increasingly expected to tackle novel challenges posed by innovative technologies, such as artificial intelligence and digital health solutions.
In this article, CareerInPharma looks at a day in the life of a regulatory affairs specialist in the pharma world, outlining some of the key benefits of this career as well as the essential requirements needed to succeed in this field.
Key Coordinators of Pharmaceutical Regulatory Affairs Specialists
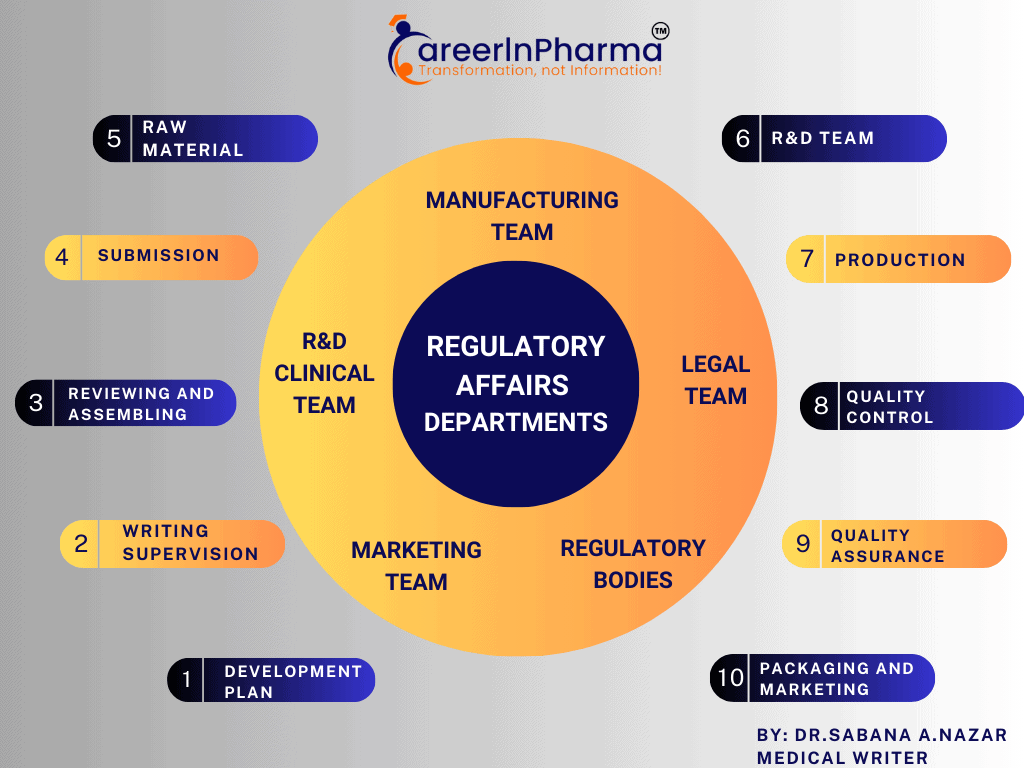
Main Responsibilities of a Regulatory Affairs Specialist
A pharmaceutical regulatory affairs specialist’s duties are determined by a variety of factors. The very common responsibilities include:
- Staying up to date with regulatory rules and guidelines.
- Maintaining, coordinating and supporting the preparation of regulatory documents for submissions includes applications for product approvals, licences, and permits.
- Reviewing and approving product labelling, packaging, monographs, and promotional materials for compliance.
- Identifying areas of weakness and implementing corrective actions based on internal audits.
- Providing guidance on regulatory strategy to the team and interacting with regulatory agencies during audits, inspections, and communications.
- Participating in the development of clinical trial regulations, protocols, and reports, thus able to evaluate and report adverse events and product complaints as required.
- Assisting in the creation and maintenance of quality assurance and control processes.
- Ensuring post-market compliance through ongoing surveillance and assessment.
- Keeping up to date on new and existing rules that may affect their company’s products and processes.
- Recommending remedial plans and applying that knowledge to standardise all business processes and create clear, written rules.
- Whenever necessary, explaining regulations, procedures, and policies to all employees and stakeholders.
Main Benefits of Working as a Regulatory Affairs Specialist
Working as a pharmaceutical regulatory affairs specialist provides you with stability and a unique opportunity to contribute directly to the safety of products to patients or consumers worldwide and also by learning about a variety of products and therapeutic details so you are becoming an expert and staying on top of evolving regulations and industry trends. The work is also highly collaborative, involving close interaction with teams, this cultivating strong communication and problem-solving skills for you. Also provide a rewarding and fulfilling career choice with a deserved salary.
A Day in the Life of a Regulatory Affairs Specialist
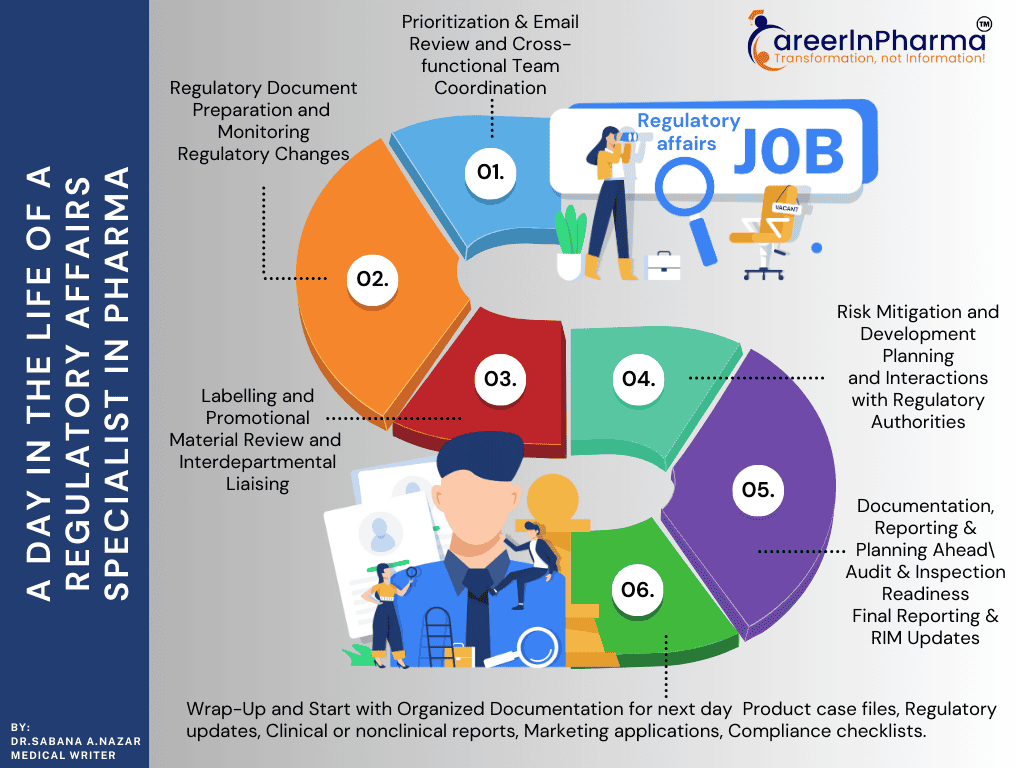
A typical day for a regulatory affairs specialist (you) entering into the office will start with morning work, including your break time with organisation, strategy & submission planning. Your work usually begins by reading emails from international regulatory agencies like the FDA, EMA, and DCGI, and internal team communications. This allows you to identify high-priority questions, compliance notifications, and future submission due dates. You are also involved in preparing and filing key applications for Clinical Trial Regulations documents, such as Clinical Trial Applications (CTAs), Investigational New Drug (IND) Applications, New Drug Applications (NDAs), or Marketing Authorization Applications (MAAs). You then attend the meetings with the research and development team, QA team, clinical teams, and medical writers to discuss clinical and nonclinical data. These meetings are focused on preparing, planning and streamlining regulatory filings and determining the best routes for product development or marketing approvals with your team, with strict adherence to ICH guidelines, norms and local regulations or legislation with your Clinical R&D team.
After your lunch break, your work focus shifts to the quality assurance (QA) team by reviewing product labels, packaging patient leaflet inserts, and promotional/educational materials for regulatory compliance. This requires coordination with the labelling team to incorporate the latest safety updates or dosing changes, ensuring materials reflect current standards as per rules and regulations. You’ll also verify the accuracy of dossiers and product monographs, which are essential for successful regulatory submissions and market approvals with the medical writers’ team. Simultaneously, you’ll examine product development plans for potential regulatory concerns and provide strategic assistance to ensure that studies have long-term objectives. You may attend meetings with the regulatory agency, where you will respond to Requests for Information (RFIs), correct submission errors or mistakes, and briefing documents to clarify or resolve outstanding unresolved concerns. These actions help you to speed approvals and also ensure that products are safe, effective, compliant, and ready for global market distribution.
As the day winds down, you finally start to wrap up pending regulatory tasks and prepare for the next day’s work. You’ll wrap up by reviewing ongoing or upcoming GMP/GCP audits and inspections, ensuring that all paperwork documentation and processes are aligned and up to date with regulatory expectations. If any regulatory compliance gaps are detected, then you’ll support the team for the creation of Corrective and Preventive Actions (CAPAs) to clear the issues proactively on the spot. You will log all updates and activities into the Regulatory Information Management (RIM) system, maintaining an accurate and traceable record. Before signing out from the office, you’ll assess the day’s work and any last-minute tasks to ensure that nothing is missed out, update internal trackers, highlight any pending or upcoming deadlines, and review the tasks scheduled for tomorrow with confidence and clarity.
Conclusion:
Therefore, joining the Regulatory Affairs course at CareerInPharma leads to a rewarding and impactful career in the pharmaceutical industry. This comprehensive program equips you with the information and hands-on skills needed to navigate global regulatory systems, facilitate medication compliance, and help public health. With the growing need for regulatory professionals, this course will make you a Regulatory Affairs Specialist and have a great impact in the healthcare field.
FAQs about Regulatory Affairs Specialist in Pharma
1. What are the key responsibilities of a regulatory affairs specialist on a typical day?
A regulatory affairs specialist ensures that pharmaceutical products are regulatory compliance by examining regulatory documentation, meeting with concerned department teams, tracking product developments, reviewing, planning, and correcting all the submissions.
2. How does a regulatory affairs specialist start their day?
The day normally starts with a review of emails from clinical research regulatory authorities (such as the FDA, EMA, and DCGI) and internal concern departments, prioritising important questions, regulatory compliance updates, and submission schedules.
3. What kind of teams do regulatory specialists work with throughout the day?
They work closely with the R&D, clinical research, quality assurance, medical writers and labelling departments to coordinate documentation, assure regulatory compliance, and strategise submissions.
4. Do regulatory affairs specialists interact with regulatory authorities directly?
Yes, they frequently take part in calls or meetings with authorities to react to RFIs, resolve issues, or plan submission tactics. They also create briefing documents and offer interpretive help to internal teams.
5. What happens at the end of the workday for a regulatory specialist?
End-of-day work includes reviewing audits or inspections, updating the Regulatory Information Management (RIM) system, evaluating pending/upcoming tasks, and monitoring for any last-minute alerts or updates.

